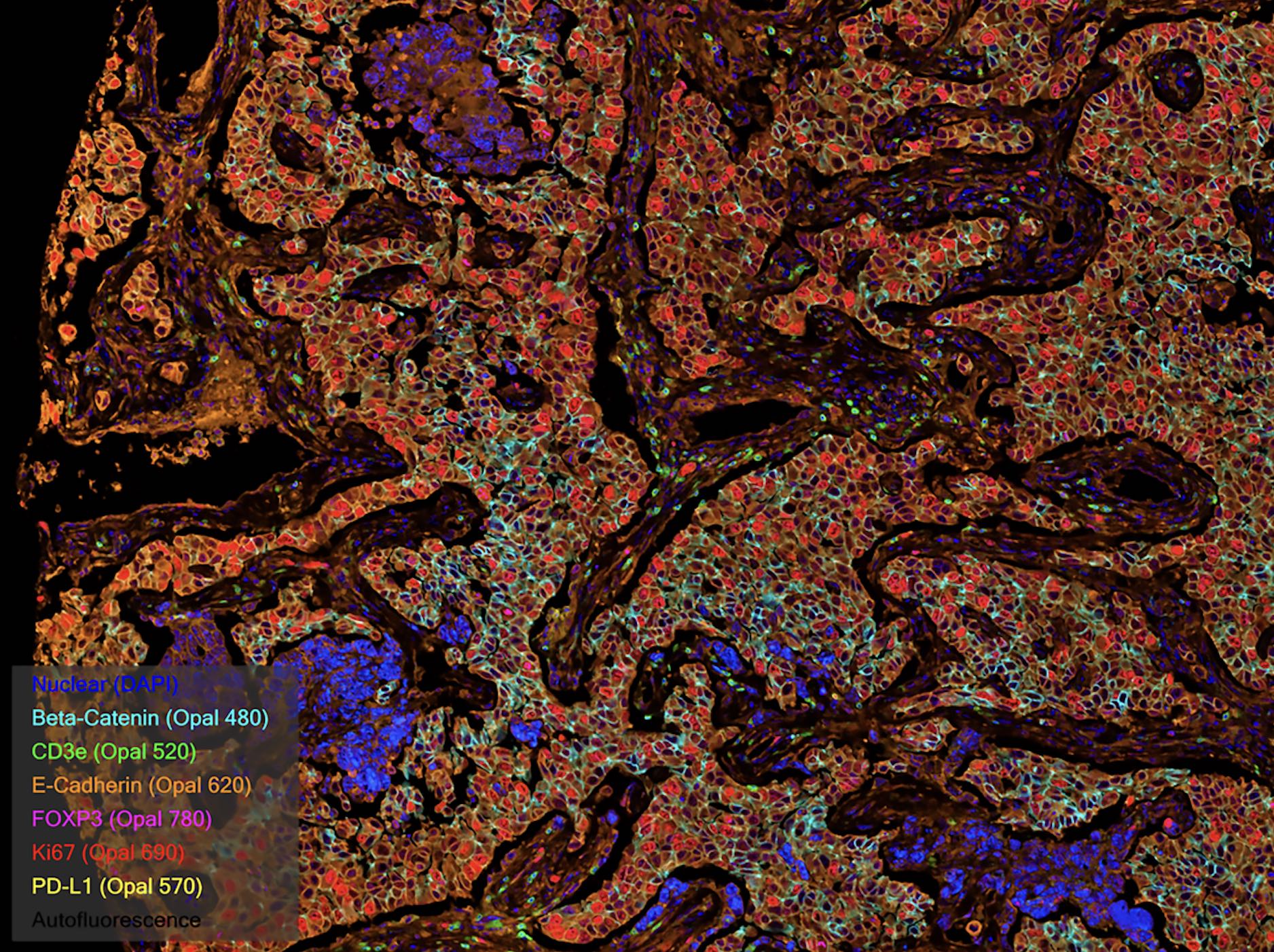
Spatial Biology
Spatial biology reveals the intricate interactions between cells and within the tissue microenvironment, which includes the extracellular matrix, immune cells, stromal cells, and the vasculature.
Multiplex Immunofluorescence
Multiplex immunofluorescence is a powerful tool within the field of spatial biology, as it allows researchers to simultaneously visualize and analyze the expression patterns and spatial distribution of numerous proteins within a tissue sample, providing valuable insights into cellular interactions, tissue architecture, and disease processes.
Multiplexing techniques use several antibodies and fluorescent detection to target specific proteins within the tissue microenvironment, allowing researchers to create a detailed map of the various cells and proteins and their interactions. In clinical settings, spatial information can be used to determine the best therapy for a particular patient. At scale, it can also assist in the identification of novel biomarkers and the development of new therapeutics based on key cell-cell interactions.
Antibodies for Multiplex IHC Spatial Biology CRO Services
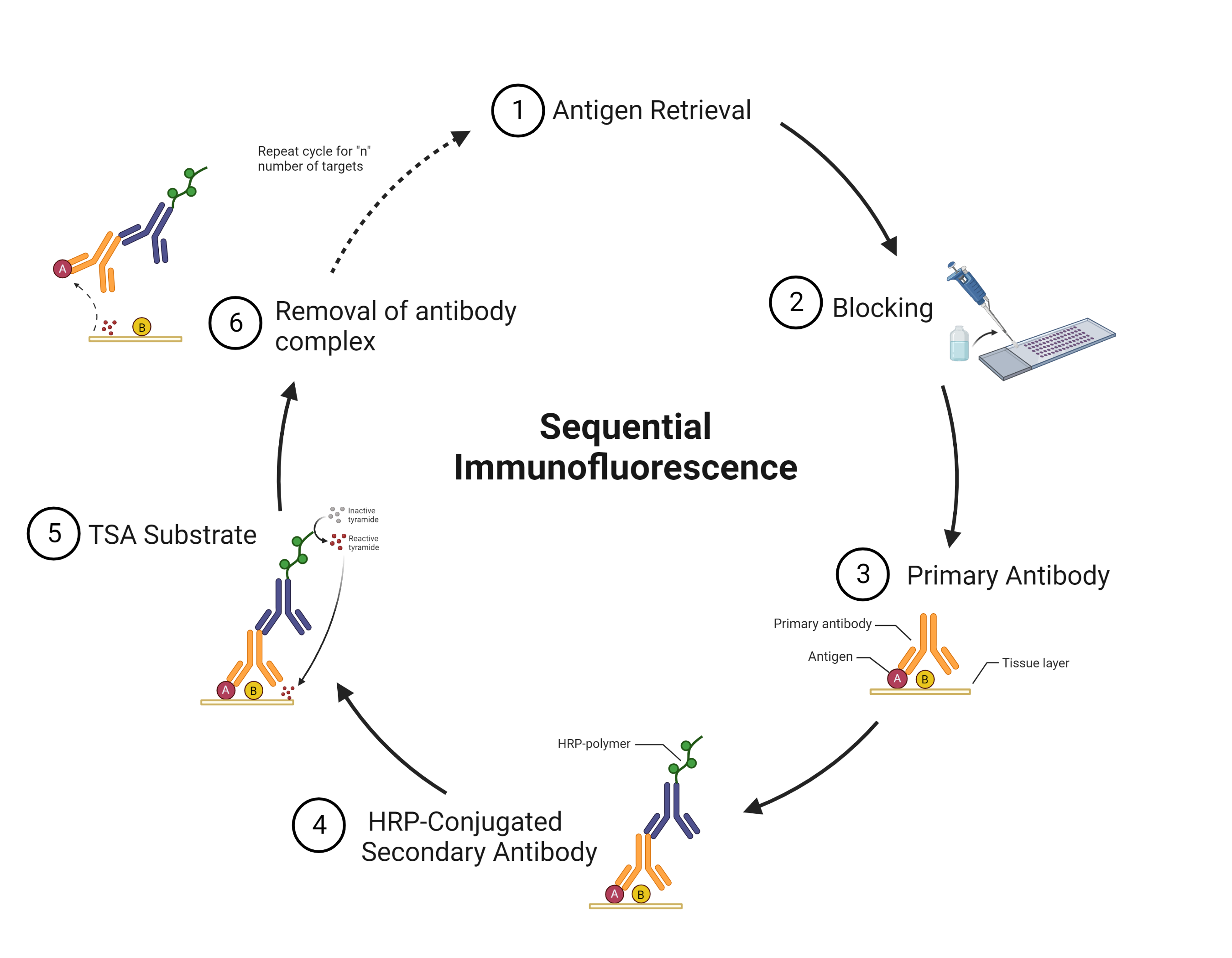
Tyramide Signal Amplification
Tyramide signal amplification (TSA) uses HRP-conjugated antibodies and hydrogen peroxide to catalyze the binding of fluorophores to proteins in the tissue sample being stained.
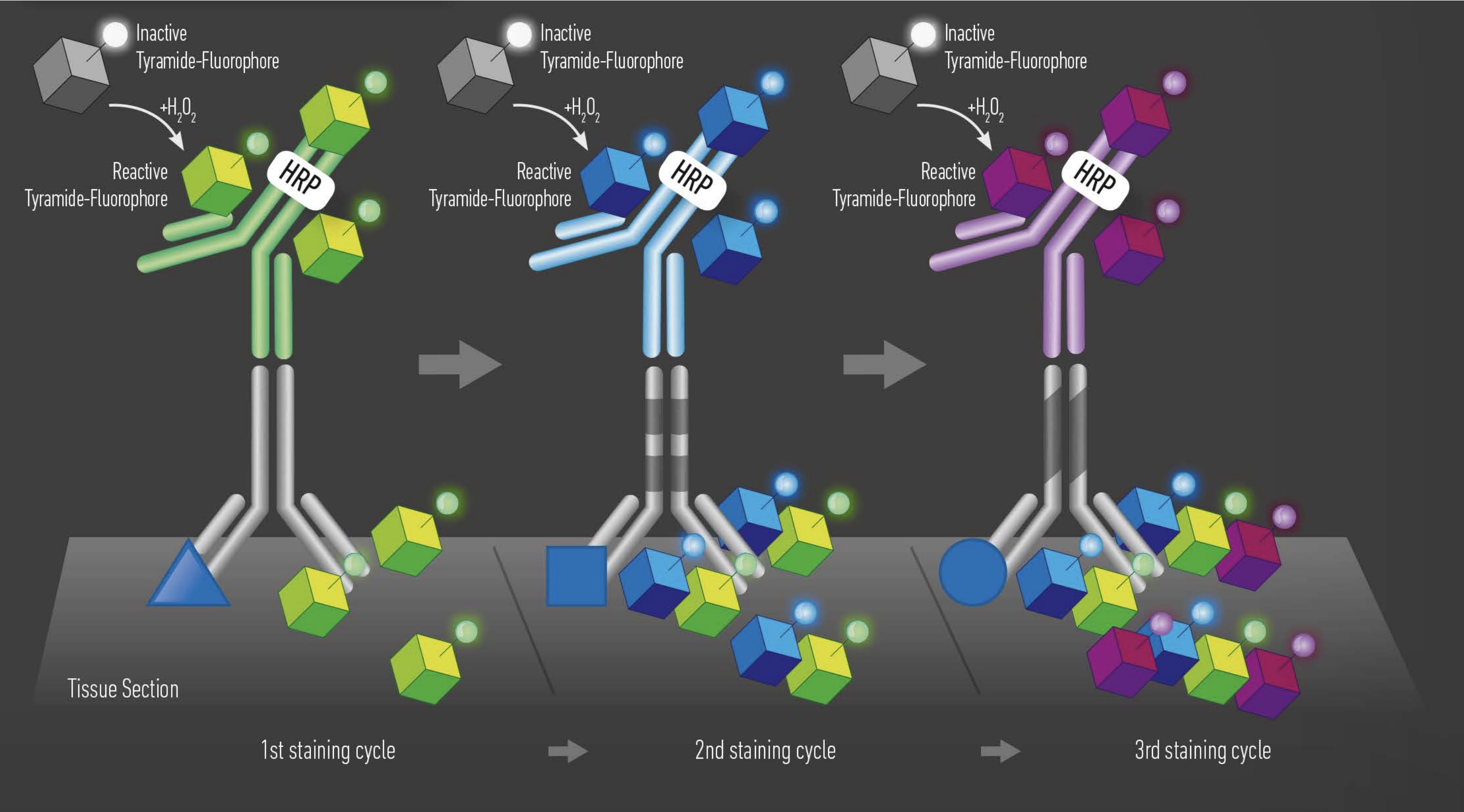
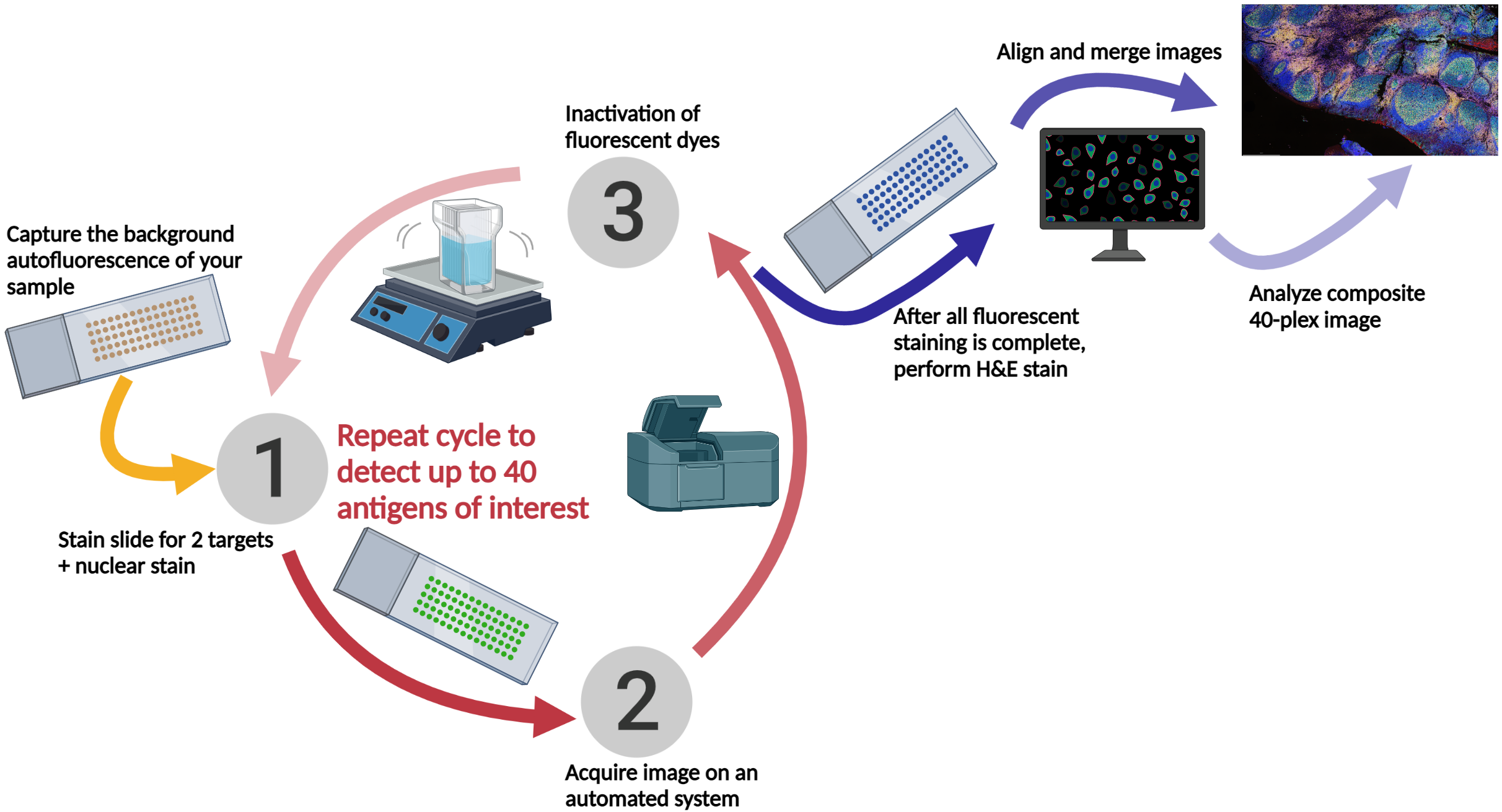
The TSA method of multiplex immunofluorescence is achieved by utilizing a basic protocol in which the primary and secondary antibodies corresponding to the first target of interest are deposited. Then, these are removed with heat-induced epitope retrieval (HIER), and the staining process is repeated for "n" number of targets.
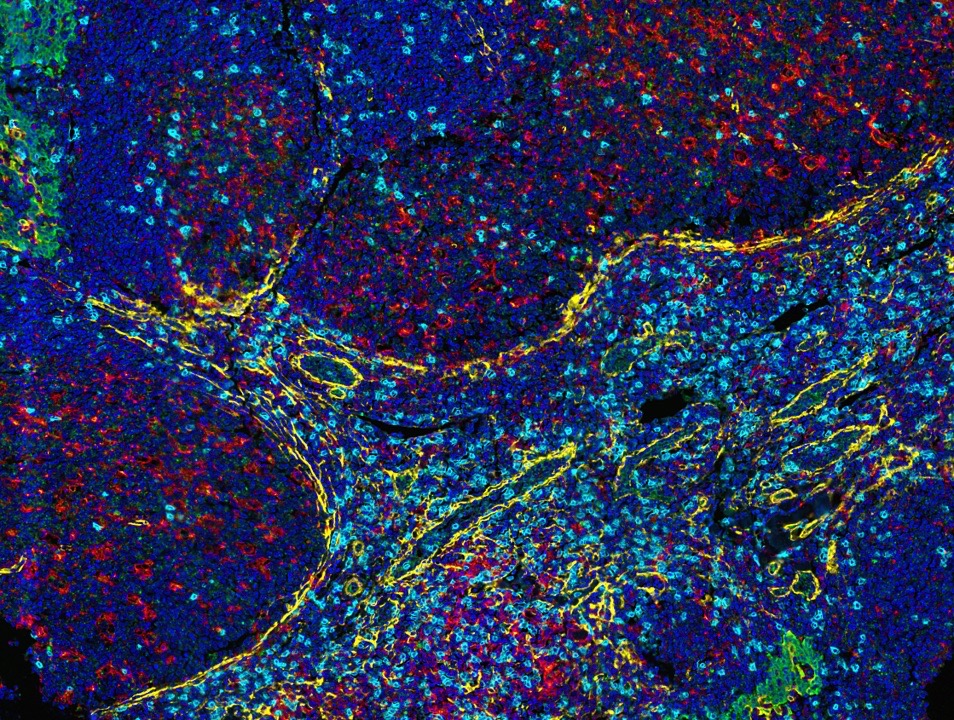
Fortis antibodies are validated for mIF with the Opal detection system from Akoya Biosciences.
Protocols
Articles
- An Introduction to Multiplex Immunohistochemistry
- Practical Overview of Multiplex Immunohistochemistry using TSA
- Benefits of Fluorescent Multiplex Immunohistochemistry using Tyramide Signal Amplification
- Evolution and Future of Multiplex Immunohistochemistry using Tyramide Signal Amplification
Resources
- Making Discoveries in Multicolor
- Bringing the Tumor Microenvironment into Focus
- Design and Optimization of Mouse Markers for Use in Melanoma, Cardiac Applications
Related Applications
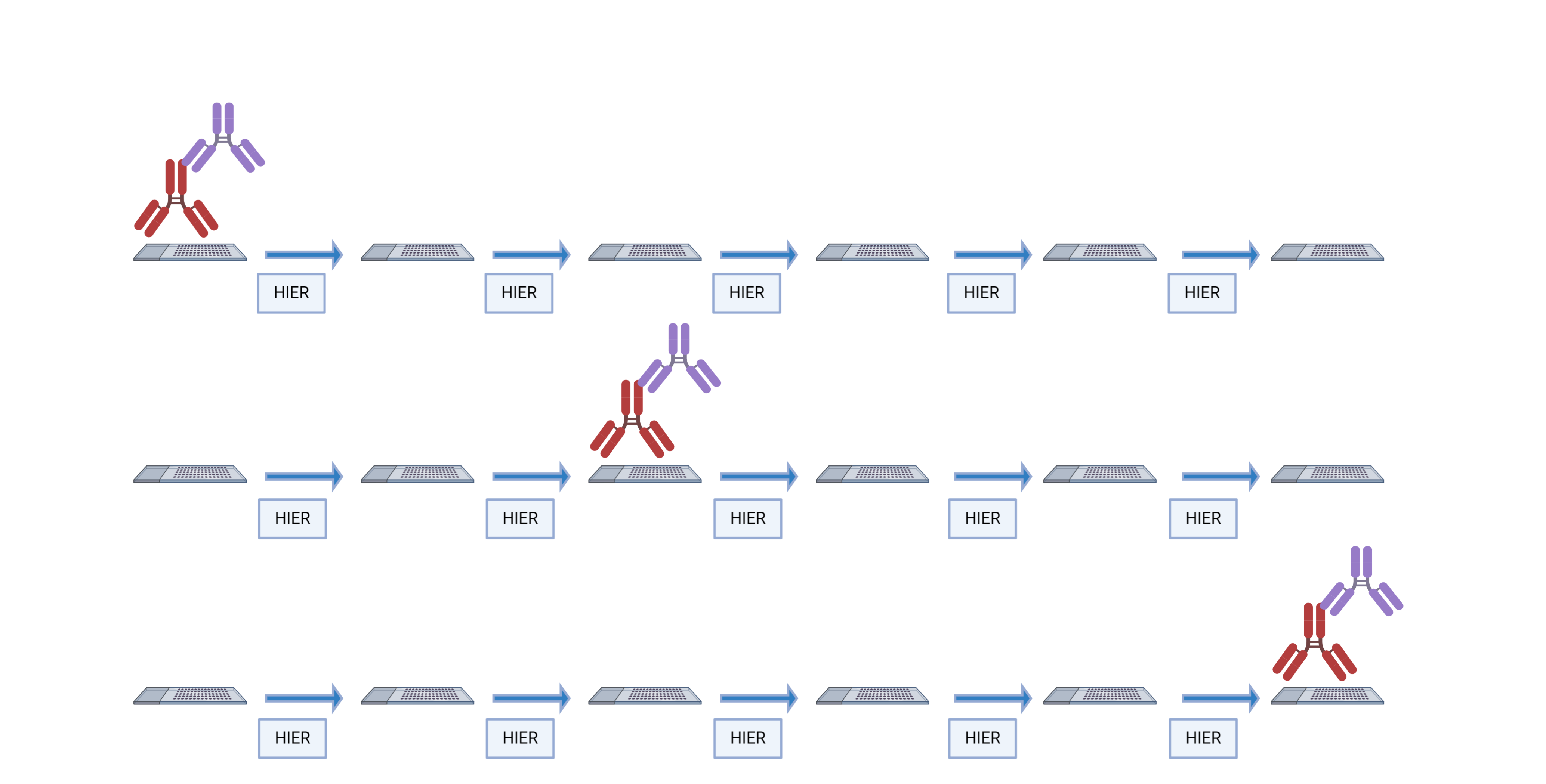
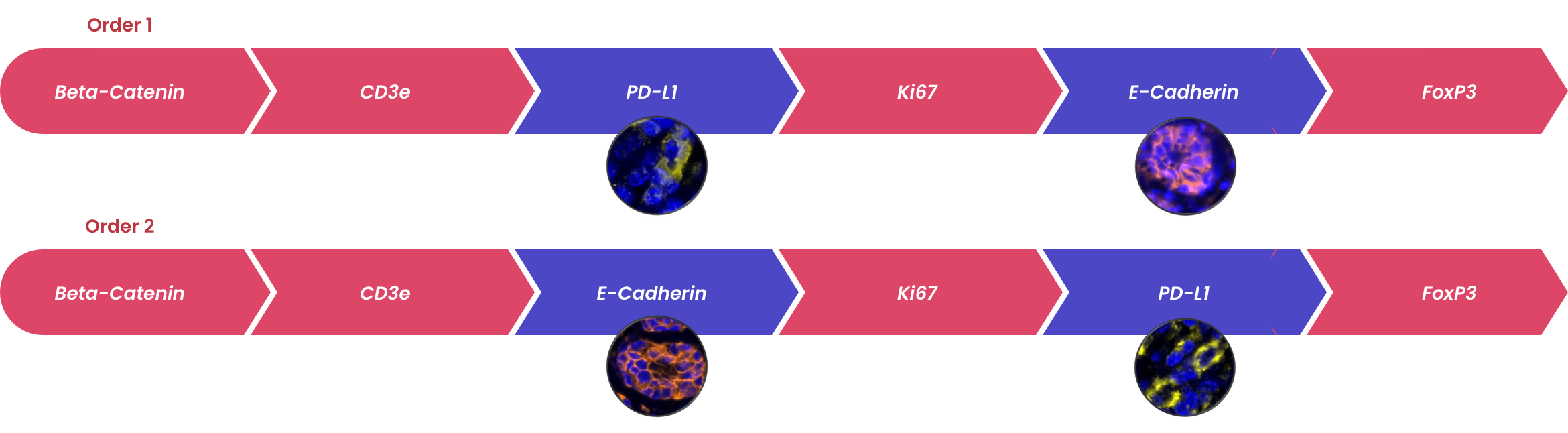
The order in which targets are applied to the tissue plays a major role in antibody activity and background intensity. So, optimizing the position that each antibody is added is the key to a successful experiment.
There are several reasons why the order of antibodies impacts the outcome of the staining:
- Repeated heating and cooling: multiple HIER steps can alter the epitope/antibody interaction.
- Tyramide blocking: tyramide deposited by previous staining steps can block the primary antibody in subsequent steps.
- Tyramide trapping or caging: deposition of tyramide may prevent the primary antibody from being removed, causing detection at subsequent staining steps.
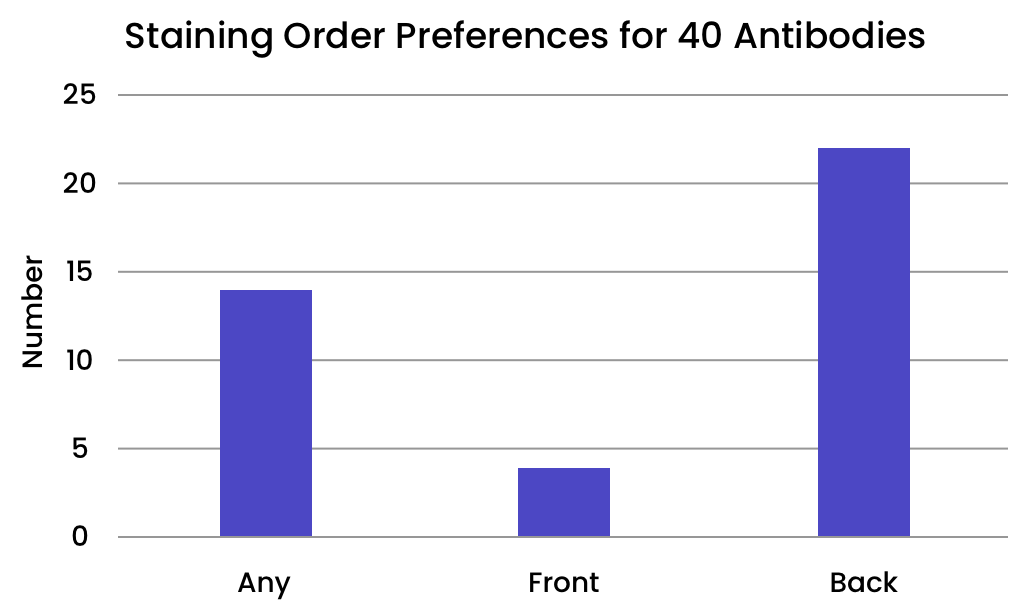
Some antibodies are more sensitive to their placement in the staining order than others. We evaluated 40 antibodies from the Bethyl catalog to determine their optimal placement in the staining order.
TSA is useful for amplifying the signal from a low-abundance protein in the tissue. The technique also produces high-resolution images because the fluorescent marker is enzymatically bound to the protein of interest.
At Fortis, we use TSA on slides produced for the Akoya PhenoImager™ HT using Opal™ reagents to generate up to 6-plex images.
Cyclic Immunofluorescence
Cyclic immunofluorescence (cyclic IF or CyCIF1 and originally referred to as MxIF2) is a high-plex imaging technique. Multiple fluorophore-conjugated antibodies are added to the slide in each round of staining. After each round of imaging, the fluorescent dyes are inactivated by chemical inactivation, photobleaching, or detergent-based stripping so that the same fluorophores can be used in subsequent rounds.2 By using the same fluorophores in multiple rounds of staining, cyclic IF minimizes the need for the use of uncommon fluorophores and extensive optimization.
The initial publication describing cyclic IF2 demonstrated that the repeated dye inactivation did not decrease epitope or tissue integrity. DAPI or Hoechst 33342 should be included in all rounds of staining to use as a reference point for image merging and analysis.
This iterative process can be performed multiple times, allowing for the detection of multiple antigens in the same tissue sample. The images acquired after each round of staining are all merged prior to being analyzed, using nuclear staining as an alignment guide.
At Fortis, we use sequential immunofluorescence (seqIF™) on the Lunaphore COMET™. It involves successive cycles of staining-imaging-elution, without any human intervention. COMET™ uniquely preserves sample morphology and epitope integrity by efficiently removing antibodies and residues from the sample. This enables samples to be reused downstream for other applications like H&E, IHC, spatial transcriptomics, or sequencing.
References
- CyCIF.org, RRID:SCR_016267
- Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue
Fortis Life Sciences and Bethyl are registered trademarks of Fortis Life Sciences.
COMET and seqIF are registered trademarks of Lunaphore Technologies S.A.
PhenoImager, Opal, and Akoya Biosciences are registered trademarks of Akoya Biosciences, Inc.
Products & Services
Our Partners

Multiplexing FAQs
mIF requires high quality IHC validated primary antibodies, HRP-conjugated secondary antibodies, tyramide detection fluorophores, and an imaging system capable of resolving the wavelengths of the fluorophores being used in the assay.
Primary antibodies of any host species can be used in mIF, even if the antibodies are from the same host species. It is advisable to begin developing mIF with highly validated IHC antibodies. Fortis Life Sciences offers an extensive portfolio of IHC validated antibodies. Antibodies from other companies are compatible with Fortis antibodies but will need to be validated by the researcher.
The most common mIF sample is formalin fixed paraffin embedded tissue (FFPE). Since mIF with tyramide detection uses multiple rounds of heat induced epitope retrieval, non-FFPE tissue tends to degrade during the assay.
Fortis antibodies are validated for mIF with the Opal detection system from Akoya Biosciences. Use of other tyramide reagents will require independent validation by the researcher.
The mIF protocol outlines the basic optimization parameters. The recommendations given for mIF applications are starting points for assay development. Further optimization of antibodies will be needed using your own tissue. Differences in tissue fixation, epitope retrieval buffer, instruments used for epitope retrieval, and technical skill can impact the mIF assay. The mIF protocol outlines the basic optimization parameters.
Order of staining can impact the detection of antigens. Multiple rounds of epitope retrieval can destroy some epitopes. In this case, antibodies recognizing these markers need to be used early in the staining order. Fortis validated mIF panels will have established an initial staining order. This order should be confirmed on your own tissue before proceeding mIF studies.
Fluorophore pairings should be carefully considered when staining for markers that occur in the same cell type, especially if those markers are localized to the same subcellular location. In these cases, it is advised to use fluorophores whose spectra are non-overlapping.
Fortis uses steamers for HIER because this method is widely used. Other methods, such as microwave treatment, may be used but will require independent validation by the researcher.
The number of targets detectable is dependent on available fluorphores and the imaging system. Fortis has platforms in our lab that allow the use of up to 40-plex assays.
Custom Request
By clicking “Acknowledge”, you consent to our website's use of cookies to give you the most relevant experience by remembering your preferences and to analyze our website traffic.